Detailed technical article about leishmaniasis.

Topics covered:
- Essentials
- Introduction
- Aetiological agent and lifecycle
- Cutaneous leishmaniasis
- Visceral leishmaniasis
- Prevention and control of cutaneous and visceral leishmaniasis
- Further reading
Essentials
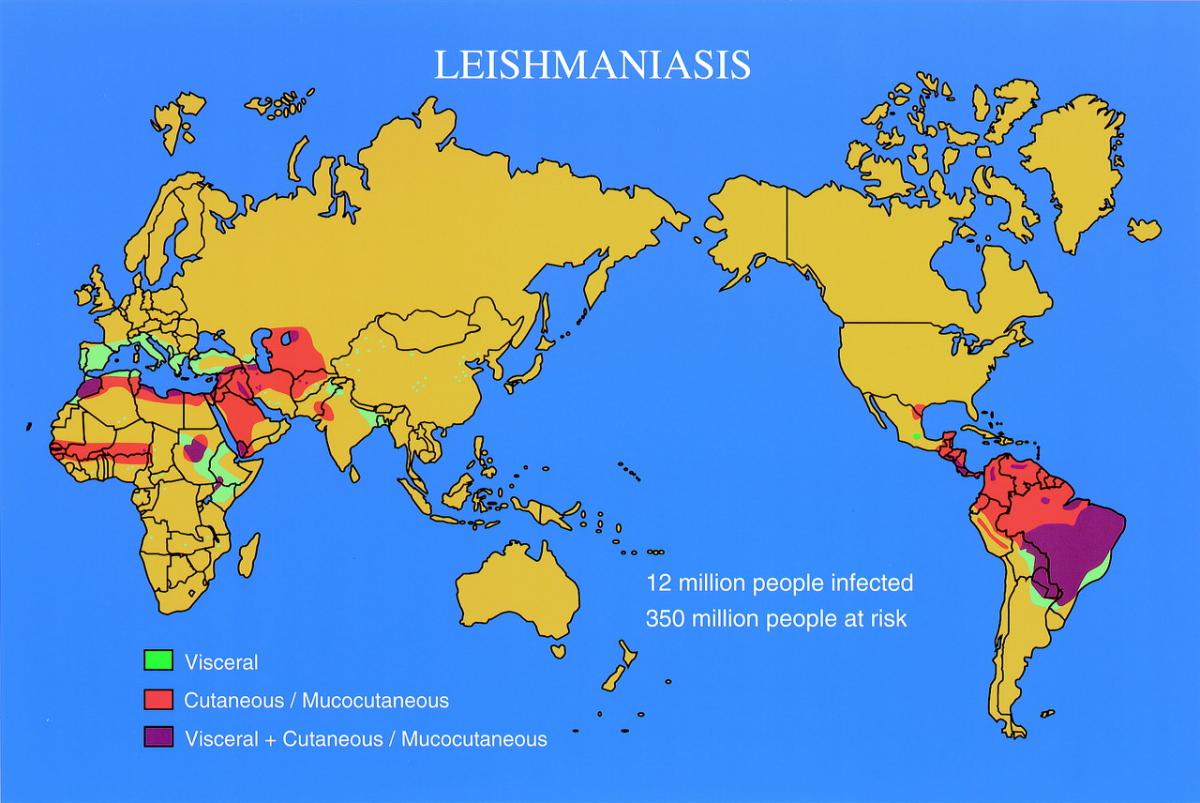
Leishmaniasis is caused by parasites of the genus Leishmania, which are transmitted to humans from human or animal reservoirs by the bites of phlebotomine sandflies. In places the disease is common and important, with perhaps 500 000 cases of visceral leishmaniasis and 1.5–2 million cases of cutaneous leishmaniasis worldwide each year. As an imported disease, cutaneous leishmaniasis is common in travellers, military personnel, and immigrants coming from endemic areas, while the diagnosis of the less common visceral leishmaniasis is frequently overlooked.
Cutaneous leishmaniasis
Clinical features—at the site of the infected sandfly bite, an erythematous nodule typically develops into a sore which fails to heal spontaneously in (1) diffuse cutaneous leishmaniasis; (2) leishmaniasis recidivans; and (3) American mucosal leishmaniasis (espundia)—a condition in which mucosal lesions develop in 4 to 40% of patients with untreated cutaneous ulcers due to L. brasiliensis; the nose is most commonly involved, and eventually the whole nose and mouth may be destroyed.
Diagnosis and treatment—diagnosis is by demonstration of leishmania organisms in tissue smears or biopsy material by microscopy, culture, or polymerase chain reaction (PCR). Many leishmanial sores can be left to heal naturally, but treatment is indicated for those that are severe, or failing to heal spontaneously, or due to particular species (e.g. L. brasiliensis).
Treatment may be (1) local—e.g. surgery/curettage; infiltration with a pentavalent antimonial; or (2) systemic—most cutaneous species of leishmania are sensitive to pentavalent antimonials.
Visceral leishmaniasis
Zoonotic disease is common around the Mediterranean littoral, across the Middle East and central Asia, in northern and eastern China, and in South and Central America. Anthroponotic disease causes large outbreaks in North Eastern India and the Sudan.
Clinical features—most infections are subclinical, but clinical presentation is with gradual onset of fever, discomfort from an enlarged spleen, abdominal swelling, weight loss, cough, or diarrhoea. The illness may be associated with HIV infection.
Diagnosis and treatment—diagnosis is by isolation of leishmania from spleen, bone marrow, liver, lymph node, or buffy coat. Serology is useful for diagnosis, and may replace direct demonstration of parasites in remote areas. The best treatment is intravenous liposomal amphotericin B, but (much cheaper) pentavalent antimonials are most often used in countries where visceral leishmaniasis is endemic.
Prevention
Prevention is by controlling reservoir hosts and sandfly vectors, or by avoiding bites by vectors. There is no vaccine.
Introduction
Leishmaniasis is caused by parasites of the genus Leishmania, which are transmitted by phlebotomine sandflies. The infection may be anthroponotic or zoonotic, having respectively human or animal reservoirs. In humans, the disease is usually either cutaneous or visceral. The most important variant is mucosal leishmaniasis of South and Central America. In places the disease is common and important, but there are few accurate statistics. The World Health Organization (WHO) estimates 500 000 cases of visceral leishmaniasis and 1.5 to 2 million cases of cutaneous leishmaniasis occur annually, with 200 million people at risk of each disease, but these figures may underestimate the problem. As an imported disease, cutaneous leishmaniasis is common in travellers, military personnel, and immigrants coming from endemic areas, while the diagnosis of the less common visceral leishmaniasis is frequently overlooked.
Aetiological agent and lifecycle
In its vertebrate host, the oval amastigote form of the parasite (2–3 μm in diameter) is found in cells of the reticuloendothelial system. In the sandfly or in culture medium, it is in the elongated, motile, promastigote form with an anterior flagellum.
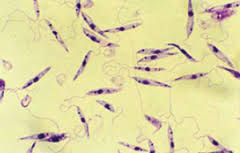
Above: leishmania promastigotes
The most important species of Leishmania that cause disease in humans and their own reservoir hosts are shown in Table 1. Isoenzyme patterns and DNA hybridization are used to distinguish species.

Above: Fluorescence stain of Leishmania mexicana promastigote
Sandflies require a precise microclimate that is provided in certain places in each endemic focus at particular seasons of the year. Transmission is often seasonal. Amastigotes are ingested from blood or tissues of the mammalian host by the female fly and transform into promastigotes in the gut, rendering the fly infective after about 10 days.
Cutaneous leishmaniasis
Epidemiology
The vectors of Leishmania major live in rodent burrows. Visiting hunters, travellers, soldiers, and tourists, and dwellers at oases or in new settlements, are affected. The disease may be sporadic or epidemic, as recently among Afghan refugees in camps in Pakistan. The vectors of L. tropica live in crevices in buildings and walls. The disease may be endemic or epidemic. The vector of L. aethiopica bites people sleeping in their huts. The disease is endemic and most people are affected by early adulthood. L. infantum causes simple, self-healing skin lesions in some parts of southern Europe and North Africa. L. donovani causes post-kala-azar dermal leishmaniasis (PKDL) in India.

Above: Sandfly - Phlebotomus papatasi. Female sandflies, but not males, bite mammals to feed on their blood.
In the New World, transmission is usually in the forest. L. brasiliensis, the major cause of American cutaneous and mucosal leishmaniasis, is the most widely distributed of the New World species. Its vectors are highly anthropophilic and human infection is common. Periurban and urban foci of infection are increasing. Malnutrition is a risk factors for mucosal leishmaniasis. Infection with L. peruviana occurs in high Andean valleys, where it may be locally common.
Pathogenesis and pathology
Leishmania, when inoculated by the sandfly, invade and multiply in macrophages in the skin.

Above: Leishmania sp. amastigotes in a Giemsa-stained tissue scraping
The parasitized macrophage granuloma is infiltrated by lymphocytes and plasma cells. Piecemeal or focal necrosis destroys parasitized cells. The overlying epidermis shows hyperkeratosis and ulcerates. In chronic lesions, epithelioid cells and Langhans giant cells produce a picture similar to that of noncaseous tuberculosis.

Above: Leishmania tropica amastigotes from an impression smear of a biopsy specimen from a skin lesion. In this figure, an intact macrophage is practically filled with amastigotes (arrows), several of which have a clearly visible nucleus and kinetoplast.
Rarely, the cellular immune response is suppressed and histology shows heavily parasitized macrophages with little or no lymphocytic infiltrate, characteristic of diffuse cutaneous leishmaniasis.
| Table 1 Epidemiology of leishmaniasis |
|---|
| Organism | Geographical location | Reservoir | Vector |
| Old World | |||
| L. donovani | North-east India, Bangladesh, Nepal | Humans | Phlebotomus argentipes |
| L. infantum | Mediterranean basin, Sudan, Middle East, China, central Asia | Dogs, foxes, jackals | P. perniciosus, P. major, P. chinensis, etc. |
| L. donovani (Africa) | Sudan, Kenya, Horn of Africa, ?Senegal, Gambia | ?Rodents in Sudan, ?canines, ?humans | P. orientalis, P. martini |
| L. major | Semideserts in Middle East, north India, Pakistan, North Africa, central Asia | Gerbils (especially Rhombomys, Meriones) | P. papatasi |
| L. major | Sub-Saharan savannah, Sudan | Rodents (especially Arvicanthis, Tatera) | P. duboscqi |
| L. tropica | Towns in Middle East, Mediterranean basin, central Asia | Humans, ?dogs | P. sergenti |
| L. aethiopica | Highlands of Kenya, Ethiopia | Hyraxes (Procavia, Heterohyrax) | P. longipes, P. pedifer |
| New World | |||
| L. chagasi (=L. infantum) | Most of Central and South America, especially Brazil | Dogs, foxes opossums (Didelphis) | Lutzomyia longipalpis, Lu. evansi |
| L. mexicana | Central and northern South America | Forest rodents (especially Ototylomys) | Lu. olmeca |
| L. amazonensis | Tropical forests of South America | Forest rodents (especially Proechimys, Oryzomys) | Lu. flaviscutellata |
| L. brasiliensis | Tropical forests and cultivated land throughout South and Central America | ?Forest rodents, dogs and equines | Lu. wellcomei, Lu. whitmani, etc. |
| L. guyanensis | Northern South America | Sloths (Choleopus), arboreal anteaters (Tamandua) | Lu. umbratilis |
| L. panamensis | Central America, Ecuador, Colombia | Sloths (Choleopus) | Lu. trapidoi, etc. |
| L. peruviana | West Andes of Peru | Dogs, ?rodents, ?opossums | Lu. verrucarum, Lu. peruensis |
L. aethiopica, L. mexicana, and L. brasiliensis may invade cartilage. Cartilaginous lesions are extremely chronic. L. brasiliensis, and occasionally L. panamensis or L. guyanensis, may metastasize through the bloodstream to sites deep in the mucosa of the upper respiratory tract, where they may lie dormant. After months or years a lesion develops, characterized by necrosis, vasculitis, and tissue destruction.
Immunity to a given species of leishmania is usually lifelong. Second infections occur occasionally, especially in older people or immunosuppressed.
Clinical features
After an incubation period of a few days to several months, an erythematous nodule develops at the site of the infected sandfly bite. A golden crust forms. The sore reaches its final size, usually 1 to 5 cm in diameter, over weeks or months. The crust may fall away, leaving an ulcer with a raised edge. Satellite papules are common. After months or years, the lesion starts to heal leaving a depressed, mottled scar. Any secondary bacterial infection is superficial and unimportant. The lesion is not normally painful, but may disfigure or disable if scarring is severe or over a joint. Draining lymphatic vessels may be thickened or nodular.

Above: Cutaneous leishmaniasis - Raised erythematous nodule
There are many variations on this classical pattern. Sores due to L. major form and heal rapidly (mean 3–5 months) and may be inflamed and exudative: the so-called wet or rural sore.
Sores due to L. tropica tend to be less inflamed and to heal more slowly (mean 10–14 months): the so-called dry or urban sore. Lesions due to L. infantum have an incubation period of many months and may persist over several years. In L. aethiopica infections, lesions are usually central on the face. Satellite papules accumulate to produce a slowly growing, shiny tumour or plaque that may not crust or ulcerate, taking between 2 and 5 years to heal; mucocutaneous leishmaniasis may develop, producing swelling of the lips and expansion and elongation of the nose. Leishmanial lymphangitis may accompany sores of any species but is commoner in the New World than the Old World. On occasion, hard thickened lymphatics may accompany an insignificant cutaneous lesion.
L. brasiliensis often causes deep, spreading ulcers, which heal over 6 to 24 months. Up to 15% of patients will relapse after spontaneous or therapeutic cure.

Above: Cutaneous leishmaniasis ulcerated lesion
L. mexicana lesions are commonly on the limbs or side of the face and heal in 6 to 8 months. Sores on the pinna of the ear may invade the cartilage, persist for many years, and destroy the pinna.
Three forms of cutaneous leishmaniasis do not heal spontaneously: diffuse cutaneous leishmaniasis, leishmaniasis recidivans, and American mucosal leishmaniasis.
Diffuse cutaneous leishmaniasis
This occurs with L. aethiopica and L. amazonensis infections but is rare. The primary nodule spreads locally without ulceration and secondary blood-borne lesions appear at other sites in the skin, affecting especially the face and the cooler extensor surfaces of the limbs. The eye, mucosae, viscera, and peripheral nerves are spared, which differentiates it from lepromatous leprosy. The infection proceeds gradually over many years.
Leishmaniasis recidivans (lupoid leishmaniasis)
This is a rare complication of L. tropica infection. The initial sore heals, but papules recrudesce in the edge of the scar and the lesion spreads slowly over many years.
American mucosal leishmaniasis (espundia)
Depending on the geographical location, between 4 and 40% of patients with untreated cutaneous ulcers due to L. brasiliensis develop mucosal lesions, half of them within 2 years of the appearance of the original lesion and 90% within 10 years. About one in six patients gives no history of a previous skin lesion. In most cases the nasal mucosa is affected, and in one-third another site is also involved: in order of frequency, the pharynx, palate, larynx, or upper lip. The initial lesion is a nodule and the initial symptom is of nasal obstruction. It commonly presents as protuberant new growth of the nose or lips, or cicatrization, which causes an elongated ‘tapir’ nose.
Mucosal leishmaniasis is slowly destructive, the septum perforates, and eventually the whole nose and mouth may be destroyed. Death may result from secondary sepsis, starvation, or laryngeal obstruction. Mucosal leishmaniasis is occasionally seen in travellers returning from South America.
Mucosal lesions are occasionally seen with Old World species, usually in the mouth or larynx, and tend to be associated with old age, corticosteroid medication, or other forms of mild immunosuppression.
Laboratory findings
Parasitological diagnosis
Normally, leishmania organisms may be isolated from 80% of sores during the first half of their natural course. The nodular part of the lesion is grasped firmly between the finger and thumb until it blanches. An incision a few millimetres long is made into the dermis with the point of a scalpel, which is used to scrape dermal tissue and juice. Material obtained may be used to inoculate special diphasic culture medium and to prepare smears for staining with Giemsa, Wright’s, or Leishman’s stains. Biopsy material may be used to make impression smears, for culture and for histology for differential diagnosis. Polymerase chain reaction (PCR) using kinetoplast DNA primers is nearly 99% sensitive and 93% specific. Diagnosis of mucosal leishmaniasis requires a deep punch biopsy specimen. Species diagnosis by PCR is desirable for American parasites to assess the risk of mucosal leishmaniasis.
Immunological diagnosis
The leishmanin test is occasionally useful in differential diagnosis. It is an intradermal test of delayed hypersensitivity that indicates previous exposure to leishmanial parasites. It becomes positive in over 90% of cases of self-healing forms of cutaneous leishmaniasis and mucosal leishmaniasis. Evaluation of a positive test must take into account naturally acquired positivity in the population at risk. Serology is unhelpful.
Treatment
Old World sores, or those due to L. mexicana, L. amazonensis, and L. peruviana that are not troublesome, may be left to heal naturally. But those that are disfiguring, potentially disabling, inconvenient, or around the ankle where they heal slowly, should be treated either locally or systemically. Systemic treatment is required when there is risk that the sore may be due to L. brasiliensis, L. panamensis, or L. guyanensis, when the sore is too large or badly sited for local treatment, when there is lymphatic spread, and for mucosal leishmaniasis, diffuse cutaneous leishmaniasis, and recidivans leishmaniasis.
Local treatment
Surgery, curettage, CO2 laser, and cryotherapy are effective methods of removing small sores. Infiltration into the lesion with a pentavalent antimonial, weekly for 2 or 3 weeks or longer, may be successful. The technique needs practice and the infiltration is transiently painful. An ointment containing 12% paromomycin and 15% methylbenzethonium chloride cures 70% lesions due to L. major in 20 days and may be suitable for lesions caused by other species, except L. brasiliensis, but is not always well tolerated.
Systemic treatment
All cutaneous species of leishmania are sensitive to pentavalent antimonials in conventional dosage except L. aethiopica, where pentamidine or paromomycin may be used. Ketoconazole may be useful for L. major and L. mexicana infections. Miltefosine is effective for L. major and L. panamensis infections. Patients with diffuse cutaneous leishmaniasis should be treated for at least 2 months, longer than it takes to clear parasites from the skin, and relapses should be treated again promptly. Relapsed cases of mucosal leishmaniasis have usually become unresponsive to antimonials and should be treated with amphotericin B deoxycholate for at least 4 to 6 weeks or liposomal amphotericin B for 3 weeks. See Tables 2 and 3 for dosage regimens. In addition, they may require antibiotics for secondary sepsis, attention to nutrition, and, later, plastic surgery.
| Table 2 Dosage regimens for the treatment of leishmaniasis |
|---|
| Drug | Dose |
| Sodium stibogluconate or meglumine antimoniate | 10–20 mg Sb/kg body weight once daily for 21 days (visceral or cutaneous disease) or 28 days (visceral or mucosal disease)—PKDL may need treatment for 2–4 months. See Table 7.8.12.3 for dosage |
| Amphotericin B desoxycholate | 1 mg/kg body weight on alternate days for 2 weeks (visceral disease) or 4–6 weeks (mucosal disease) |
| Liposomal amphotericin B | Ampoules of 50 mg, 2–3 mg/kg body weight daily for 7–10 doses, using whole ampoules to avoid waste, to total at least 21 mg/kg. In India a total dose of 6–9 mg/kg is sufficient. A 20-day regimen cures PKDL in Sudan |
| Miltefosine | Adult dose 100–150 mg daily for 28 days; paediatric dose 2.5 mg/kg body weight daily for 28 days |
| Aminosidine | 16 mg/kg body weight daily for 21 days |
| Pentamidine | 4 mg salt/kg body weight once weekly to once monthly |
| Ketoconazole | 60 mg/day (adult) for 4–6 weeks |
|
See text for choice of drug regimen. |
| Table 3 Simplified dosage regimens for pentavalent antimonials |
|---|
| Nearest weight of patient (kg) | Calculated dose (mg Sb) | Recommended dose (ml (mg Sb)) | |
| Sodium stibogluconatea | Meglumine antimoniateb | ||
| 90 | 1088 | 11.0 (1100) | 13.0 (1105) |
| 80 | 1006 | 10.0 (1000) | 12.0 (1220) |
| 70 | 925 | 9.5 (950) | 11.0 (935) |
| 60 | 832 | 8.5 (850) | 10.0 (850) |
| 50 | 737 | 7.5 (750) | 9.0 (765) |
| 40 | 635 | 6.5 (650) | 7.5 (637) |
| 30 | 524 | 5.0 (500) | 6.0 (510) |
| 20 | 400 | 4.0 (400) | 5.0 (425) |
| 10 | 252 | 2.5 (250) | 3.0 (255) |
| 5 | 159 | 2.0 (200) | 2.5 (212) |
|
Calculations are based on body surface area according to the formula: body surface area in m2 = 0.13/kg2, whereby a 20 kg child receives 20 mg Sb/kg at 542 mg Sb/m2. a Sodium stibogluconate solution containing 100 mg Sb/ml. b Meglumine antimoniate solution containing 85 mg Sb/ml. (Adapted from Anabwani GM, Bryceson AD (1982). Visceral leishmaniasis in Kenyan children. Indian Pediatr, 19, 819–22.) |
Visceral leishmaniasis
Epidemiology
Visceral leishmaniasis is found in four main zoogeographical zones: the Ganges Brahmaputra plains, the Mediterranean basin extending into West and Central Asia, Sudan and East Africa, and Brazil (see Table 1).
Around the Mediterranean littoral, across the Middle East and central Asia, and in northern and eastern China, zoonotic visceral leishmaniasis is endemic in many places, where as many as 50% of domestic and stray dogs may be infected. Children under 5 years of age are especially affected. It is the second most common infectious cause of fever of unknown origin in children in the Balkan countries. HIV infection is a risk factor for adults. In other places, the disease is sporadic. Nonimmune adults such as tourists, hunters, and soldiers are susceptible.
The Ganges and Brahamputra river valleys of India and Bangladesh are the home of epidemic anthroponotic visceral leishmaniasis, or kala-azar, which returns approximately every 15 to 20 years. The majority of cases are in young people under 15 years of age and are found in clusters. The annual incidence is about 250 per 100 000. About 50% of household contacts of cases in Bihar India are seropositive, one in four of whom will develop disease. Malnutrition predisposes to clinical disease. In the interepidemic period, the parasite survives in patients with post-kala-azar dermal leishmaniasis.
Visceral leishmaniasis is endemic in parts of Sudan, where it may be both anthroponotic and zoonotic, and in adjacent parts of Ethiopia and Kenya. Older children and teenagers are most commonly affected. Sporadic cases also occur in nomads and visitors. In Sudan, an epidemic that began in the south in the late 1980s and caused over 100 000 deaths between 1984 and 1994 is still raging. It has been especially severe among refugees from the civil war. In remote areas, half the cases do not reach a medical facility and 90% of deaths go unreported.
In South America, the disease is most common in north-eastern Brazil, where older children are affected. Previously a rural disease, it is becoming increasingly important in towns.
Visceral leishmaniasis may appear unexpectedly in immunosuppressed patients, e.g. after renal transplantation, with haematological malignancies, while receiving immunosuppressive drugs, and in pregnant women. In endemic areas, it is an opportunistic infection in patients with HIV infection.
Visceral leishmaniasis may be transmitted by blood transfusion from subclinical cases; parasites were cultured from 2 to 4% of donor blood samples in endemic areas of France and Spain.
Pathogenesis and pathology
For every case of classical visceral leishmaniasis, there are about 30 subclinical infections that cause leishmanin positivity and lifelong immunity to the infecting species. Established visceral infections are characterized by the failure of specific cell-mediated immunity. The leishmanin test is negative. The parasite multiplies freely in macrophages in the spleen, bone marrow, lymphoid tissues, jejunal submucosa, and Kupffer cells of the liver. Histology shows a variable degree of granuloma formation and interstitial inflammation in the liver that may lead to fibrosis. In the spleen especially, there is massive reticuloendothelial hyperplasia and infiltration with plasma cells. Small splenic infarcts may develop.
Antibodies, polyclonal IgG, and immune complexes circulate at high concentration but rarely cause complications. About half of the patients have mild malabsorption but seldom diarrhoea. When present, jaundice usually has another cause such as viral hepatitis. Spontaneous bleeding is unusual and is associated with hypoprothrombinaemia. Visceral leishmaniasis is characterized by anaemia, leukopenia, thrombocytopenia, and hypoalbuminaemia. The anaemia results mainly from shortened red-cell survival with destruction of cells in the spleen, together with splenic pooling and sequestration (hypersplenism). In young children, profound anaemia may develop rapidly as a result of severe haemolysis. Death is usually due to secondary infection.
Clinical features
The male/female ratio is between 3:1 and 4:1. The incubation period is usually 2 to 8 months. In endemic areas, the onset is usually ill defined. The patient develops fever, discomfort from an enlarged spleen, abdominal swelling, weight loss, cough, or diarrhoea. Classically, the fever spikes twice daily, usually without rigors, but daily, irregular, or undulant fevers are common. During an epidemic or in visitors to an epidemic area, symptoms may start abruptly with high fever and rapid progression of illness with toxaemia, weakness, dyspnoea, and acute anaemia.
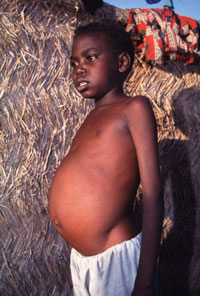
Above: Child with visceral leishmaniasis
Physical examination of early cases may show only symptomless splenomegaly. Patients with advanced disease are wasted, with hair changes and pedal oedema typical of hypoalbuminaemia. Hyperpigmentation is characteristic of visceral leishmaniasis in India (kala-azar means ‘black disease’). The spleen is huge, smooth, and nontender unless there has been a recent infarct. The liver is moderately enlarged in one-third of cases. In African patients, a generalized lymphadenopathy is common.

Above: Child with visceral leishmaniasis with hepatosplenomegaly - the spleen is massively enlarged
Over months or years the patient becomes emaciated, with a distended abdomen. Intercurrent infections are common, especially pneumococcal otitis media, pneumonia, septicaemia, tuberculosis, measles, dysentery, other locally important infections, and rarely, cancrum oris. Untreated, between 80 and 90% of patients die.
Post-kala-azar dermal leishmaniasis (PKDL)
About up to 10% of Indian patients and up to 50% of Sudanese patients develop a rash on the face, extensor surfaces of the arms and legs, and trunk after recovery from visceral leishmaniasis. In India, the rash begins after an interval of 1 or 2 years and progresses over many years: pale macules become erythematous plaques, papules, or nodules resembling lepromatous leprosy, and almost the entire body surface may be involved. In Kenya, the rash usually appears while the patient is still recovering, as discrete nodules, which show a granulomatous histology with scanty parasites. It heals spontaneously within 6 months. Sudanese patients show a mixture of these two forms. PKDL is rarely seen after L. infantum infections.
Visceral leishmaniasis and HIV infection
Visceral leishmaniasis may be associated with HIV infection, especially in southern Europe, where it is commonest among intravenous drug users. It may be due to reactivation of latent infection with Leishmania or to a recent infection. In Spain, over 50% of adults with visceral leishmaniasis are HIV positive, and it is estimated that 9% of HIV-infected people will acquire visceral leishmaniasis. In northern India, during 2004/05, about 6% of all cases were coinfected with HIV. The presentation may not be typical and there may be unusual skin lesions. Antiretroviral treatment has greatly reduced the clinical impact of coinfection, but in some patients leishmaniasis now presents as an immune reconstitution inflammatory syndrome. Often the parasite is found by chance, e.g. in a rectal or skin biopsy taken for other purposes, or in bronchoscopic lavage. The bone marrow is teeming with parasites but two-thirds of cases have no detectable antileishmanial antibodies. In 90% of cases, the CD4 count is less than 0.2 × 106/litre. Response to treatment is poor and relapse usual (see ‘Treatment’ below). HIV coinfected people are infective to sandflies and may also transmit parasites by sharing needles.
Laboratory diagnosis
Parasitological diagnosis
Leishmania organisms may be isolated from reticuloendothelial tissue. Yields are of the order of: spleen, over 95% cases; bone marrow or liver, 85%; lymph node in Sudan, 65%; and buffy coat, 70%. Bone marrow aspiration is most commonly used, but splenic aspiration is simple, painless, and safe if the prothrombin time is normal and the platelet count above 40 × 109/litre. Occasionally, the diagnosis is made accidentally on biopsy of bone marrow, liver, lymph node, or bowel mucosa. PCR for leishmanial DNA in bone marrow is even more sensitive. PCR for leishmanial DNA in blood is useful for follow up HIV co-infected patients.
Serological diagnosis
Antibodies are present in high titre, useful for diagnosis, and may replace parasite diagnosis in the remote areas. Indirect immunofluorescence is the gold standard but, for fieldwork, it has been replaced by enzyme-linked immunosorbent assay, direct agglutination, and the rK39 antigen dipstick. All give comparable results with sensitivities of about 90% and specificities above 95% (positive predictive value c.99% and negative predictive value c.70%). The leishmanin skin test is negative.
Other findings
There is normochromic, normocytic anaemia without reticulocytosis, and neutropenia, eosinopenia, and thrombocytopenia. Serum albumin is low (c.20 g/litre) and globulin high (c.70 g/litre), IgG and IgM being approximately thrice and twice the normal population values. Hepatic enzymes and prothrombin and partial thromboplastin times are usually normal.
Treatment
Chemotherapy
Liposomal amphotericin B by intravenous infusion is the best drug for visceral leishmaniasis in adults and children. It is concentrated and retained in reticuloendothelial cells and is not toxic. Over 99% patients respond promptly, but HIV-coinfected patients relapse. The drug is also effective against PKDL in India and Sudan and it is the drug of choice in pregnancy. At the moment, it is far too costly for most countries where visceral leishmaniasis is endemic, but World Health Organization has recently negotiated a 90% reduction in price. Therefore, a pentavalent antimonial remains the drug of choice in most situations. See Tables 2 and 3 for dosage regimens.
Conventional amphotericin B deoxycholate is cheaper than liposomal amphotericin B and just as effective, though more toxic, and is useful for patients unresponsive to antimonials.
Sodium stibogluconate containing 100 mg Sb/ml and meglumine antimoniate containing 85 mg Sb/ml are of equal efficacy and toxicity. The drug is administered by intramuscular injection, which may be painful, or by intravenous injection through a fine-gauge needle, slowly or by infusion in 50 to 100 ml of 5% dextrose over 20 min to reduce the risk of venous thrombosis. Treatment is given daily for 28 days. Usually the drug is well tolerated but towards the end of treatment there may be malaise, anorexia, nausea, vomiting, and muscle pains. Should toxic effects develop, rest for 1 day and reduce each dose by 2 mg Sb/kg. Hepatic and pancreatic enzyme levels may rise and haemoglobin levels fall, but they return to normal when treatment is stopped. The electrocardiogram develops unimportant T-wave changes. At higher doses, the corrected QT interval may be prolonged, heralding the development of a serious arrhythmia. Cure rates exceed 95% except in Bihar, north of the river Ganges where primary antimony resistance is spreading and up to 60% patients do not respond to antimonials. Secondary resistance develops in patients who relapse.
The aminoglycoside antibiotic paromomycin, or aminosidine, is equally effective and well tolerated, but cure rates vary between countries. It is given by intramuscular injection or intravenous infusion over 90 min. Renal function and hearing should be monitored.
A new oral drug, miltefosine, cures from 90 to 94% of HIV-negative adults and children with visceral leishmaniasis in Sudan and India, even in areas of parasite resistance to antimonials.
Patients who are immunosuppressed as a result of HIV coinfection or immunosuppressive drugs respond slowly, require longer treatment, and are more liable to relapse than immunocompetent patients. Ideally, treatment of such patients should be monitored by splenic aspirate counts of parasites and continued for 2 or 3 weeks beyond parasitological cure. Antimonials cause adverse effects in two-thirds of HIV coinfected patients and may cause pancreatitis. Liposomal amphotericin B and aminosidine are effective and well tolerated. Relapse may be prevented by secondary prophylaxis with pentamidine given every 2 weeks. Highly active retroviral therapy (HAART) reduces the number of relapses and delays their onset.
Supportive treatment
Intercurrent infection must be sought and treated and nutritional deficiencies corrected. Blood transfusion is rarely needed.
Response to treatment
Fever, splenic size, haemoglobin, serum albumin, and body weight are useful monitors of progress. Proof of parasitological cure is not usually necessary. Reassessment at 6 weeks and 6 months will detect over 90% of relapses. Serology is unhelpful in monitoring progress. Relapse rates should be under 4%. Relapsed patients are slower to respond and run a 40% chance of further relapses and of becoming unresponsive to antimony. Primary parasite resistance to antimonials is increasing in India where the next choice lies between miltefosine, aminosidine and amphotericin B deoxycholate. Treatment with two drugs might prevent parasite resistance, and combinations are being tested.
Economic impact
Visceral leishmaniasis is a major economic burden on affected families. The direct costs of an episode of visceral leishmaniasis in rural India or Bangladesh, where the drug is, in principle, provided free, are equivalent to the household’s annual income.
Prevention and control of cutaneous and visceral leishmaniasis
Prevention is a matter of controlling reservoir hosts and sandfly vectors or of avoiding bites by vectors. Successful control requires an accurate knowledge of transmission in each ecological focus.
In the Old World, urban cutaneous leishmaniasis is controlled by case-finding and treatment, better housing, and domestic spraying with residual insecticides, while rural leishmaniasis is controlled in the Middle East and North Africa by poisoning or destruction of gerbil colonies. Mediterranean and urban visceral leishmaniasis in South America may be controlled by the destruction or treatment of dogs, but dogs are infectious to flies before they become symptomatic and screening of dogs is problematic. Dog collars impregnated with permethrin reduce the numbers of flies that become infected. In India, mass campaigns to spray houses and cattle sheds are needed. In the interepidemic period, cases of PKDL should be sought and treated. Currently no nation has an effective control programme in place.
In endemic populations, infection may be prevented during the season of transmission by the use of insect repellent creams and fine mesh bed nets, top sheets or chadors (women’s outer garments or cloaks) impregnated with permethrin during the hours of biting, usually around dusk and dawn. In endemic foci, a higher level of education in households is associated with lower rates of disease. Vaccines have proved disappointing.
Further reading
Blum J, et al. (2004). Treatment of cutaneous leishmaniasis among travellers. J Antimicrob Chemother, 53, 158–66.[Abstract/Full Text]
Cruz I, et al. (2006). Leishmania/HIV co-infections in the second decade. Indian J Med Res, 123, 357–88. [Web of Science] [Medline]
den Boer M, Davidson RN (2006). Treatment options for visceral leishmaniasis. Expert Rev Anti Infect Ther, 4, 187–97.[CrossRef] [Medline]
Desjeux P (2001). The increase in risk factors for leishmaniasis worldwide. Trans R Soc Trop Med Hyg, 95, 239–43.[CrossRef] [Web of Science] [Medline]
Lockwood DNJ, Sundar S (2006). Serological tests for visceral leishmaniasis. Br Med J, 333, 711–12.[Abstract/Full Text]
Murray HW, et al. (2005). Advances in leishmaniasis. Lancet, 366, 1561–77.[CrossRef] [Web of Science] [Medline]
Websites
Centres for Disease Control. http://www.cdc.gov/parasites/leishmaniasis/index.html
World Health Organization. Leishmaniasis. http://www.who.int/leishmaniasis
[Both have good summaries on leishmaniasis and links to new research findings.]